Implen Journal Club | September Issue Innovations in Technology |
|
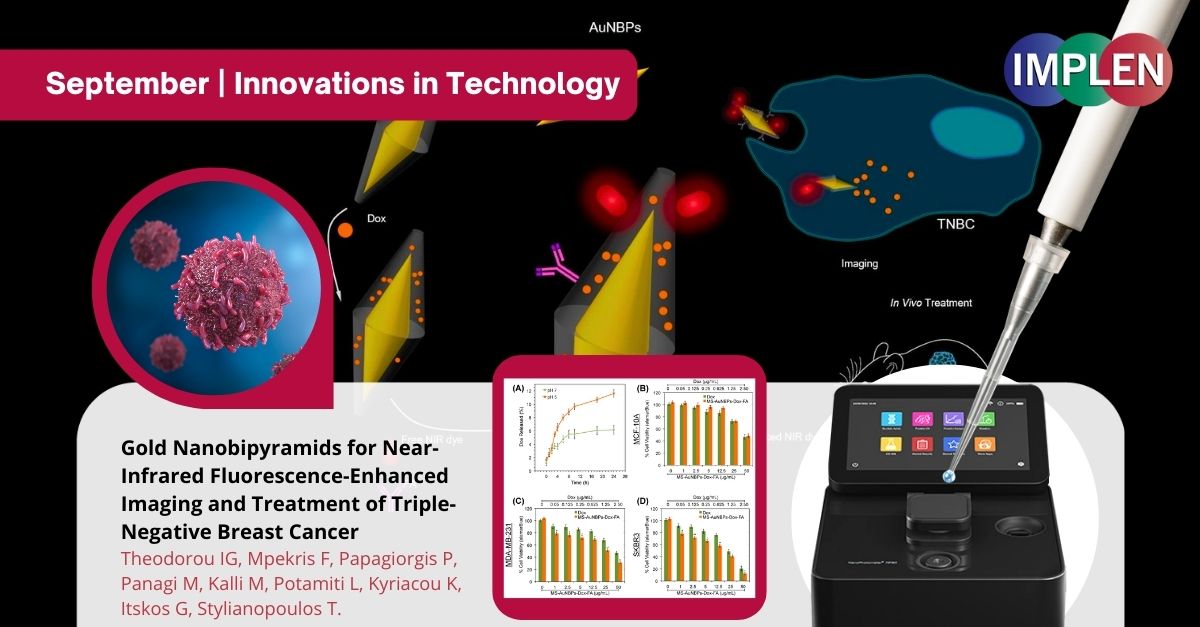
The first issue of the Implen NanoPhotometer® Journal Club is highlighting a significant advancement in the field of breast cancer research, specifically targeting Triple-Negative Breast Cancer (TNBC) published recently by Theodorou et. al. in the journal Cancers. TNBC is a particularly aggressive form of breast cancer that poses challenges due to the absence of specific receptor targets, making it difficult to treat effectively. The researchers aimed to address this challenge by exploring the use of Gold Nanobipyramids. Gold Nanobipyramids are minuscule structures that show promise in enhancing near-infrared fluorescence imaging for TNBC. This advancement offers improved precision and early detection capabilities, which are crucial in managing the disease effectively. The study not only focused on improving imaging techniques but also delved into the potential of Gold Nanobipyramids for targeted therapy. This means that these nanostructures may not only aid in better visualization of TNBC but also in delivering treatments directly to cancer cells, minimizing collateral damage to healthy tissue. This is promising research, offering hope for more effective diagnosis and treatment strategies, ultimately improving the prognosis for TNBC patients. This is especially significant given the aggressive nature of the disease and the limited treatment options available. This study represents a notable example of the intersection of nanotechnology and cancer treatment, emphasizing the importance of interdisciplinary collaboration in scientific progress. Such innovative approaches are essential in advancing our understanding of complex diseases like breast cancer and improving patient outcomes. The NanoPhotometer® was used in this research to characterize the gold nanobiopyramids using optical absorption spectroscopy. |
|
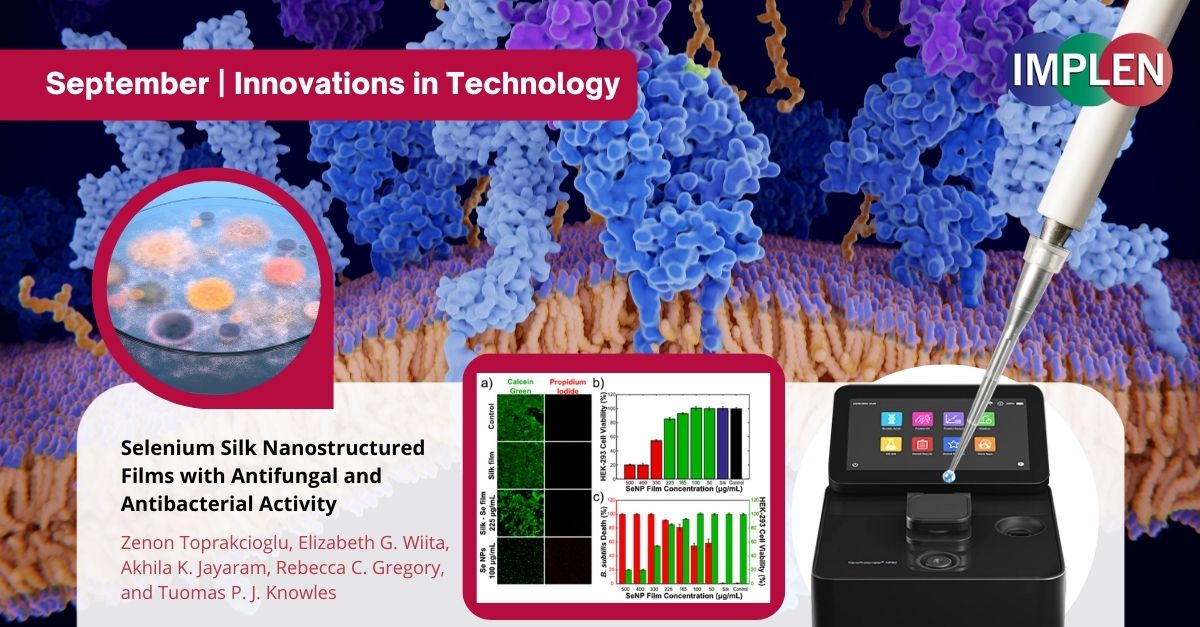
In the second issue, we explore a recent groundbreaking study by Toprakcioglu et al. with research that presents a novel solution to the urgent problem of bacterial and fungal infections through the development of selenium-infused silk films exhibiting remarkable antimicrobial properties. Selenium, known for its antimicrobial attributes and previously examined for potential therapeutic uses, was ingeniously incorporated into silk films using an environmentally friendly and straightforward technique. This method effectively preserved silk's natural biocompatibility. The outcome was the creation of selenium-infused silk films that exhibited impressive antibacterial and antifungal activities against a spectrum of pathogens. Remarkably, these films demonstrated efficacy against both Gram-positive and Gram-negative bacteria, rendering them versatile for addressing various bacterial infections. Furthermore, they displayed antifungal properties, providing a multifaceted approach to combating infections. The selenium-infused silk films were observed to disrupt the integrity of bacterial and fungal cell membranes, elucidating the mechanistic insight into their antimicrobial effects. This understanding enhances our knowledge of how such materials combat infections. This work highlights the potential of harnessing nanomaterials and natural biopolymers for the development of advanced materials with significant therapeutic value and holds promise for diverse applications, including medical devices, wound dressings, and coatings for surfaces in healthcare settings. The selenium-infused silk films present a sustainable and biocompatible solution to the critical challenge of microbial infections. The NanoPhotometer® NP80 was used in this study to characterize selenium nanoparticles (SeNP’s) using UV-vis absorption spectroscopy. The spectra were recorded within a wavelength range of 200–400 nm. |
|
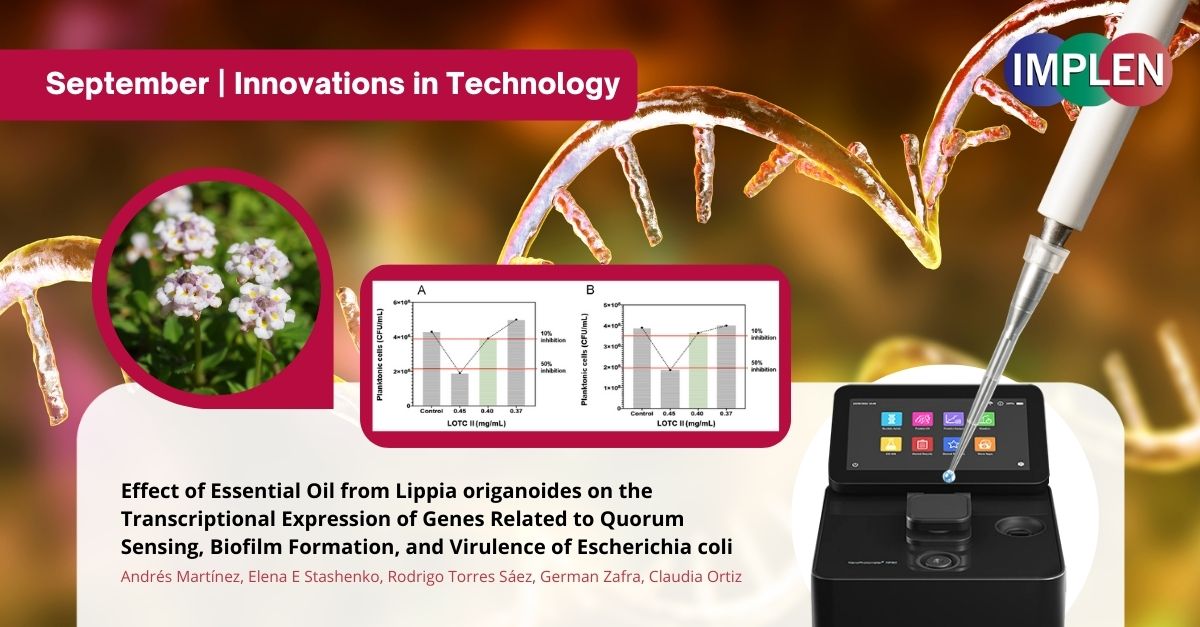
Next issue is highlighting an innovative exploration into combating bacterial infections, particularly those caused by Escherichia coli (E. coli) in a study conducted by Andrés Martínez et. al. recently published in the journal Antibiotics. This work introduces a fresh and pioneering approach to addressing antibiotic-resistant bacteria. This study harnessed the potential of Lippia origanoides essential oil, a natural compound known for its antimicrobial properties. Instead of focusing solely on its bactericidal effects and investigated how this essential oil influences the transcriptional expression of genes associated with quorum sensing, biofilm formation, and virulence in E. coli. This genetic-level analysis sets this study apart from conventional antibiotic research. By targeting critical bacterial processes, the essential oil disrupts quorum sensing, inhibits biofilm formation, and downregulates virulence-related genes. This multifaceted approach innovatively weakens E. coli's ability to cause infections. Furthermore, the research suggests that Lippia origanoides essential oil could serve as an adjunctive therapy, potentially enhancing the effectiveness of existing antibiotics. This combined approach represents an innovative strategy to combat antibiotic resistance, a growing concern in the field of infectious diseases. This study's innovative approach lies in its utilization of a natural compound, its genetic-level analysis, and its multifaceted strategy to combat E. coli infections. By disrupting crucial bacterial processes and exploring the potential of adjunctive therapy, it offers a promising avenue to address antibiotic resistance, marking a significant advancement in the fight against bacterial infections. The NanoPhotometer NP80 was used in this study to determine the concentration and purification of total RNA was spectrophotometrically. |
|
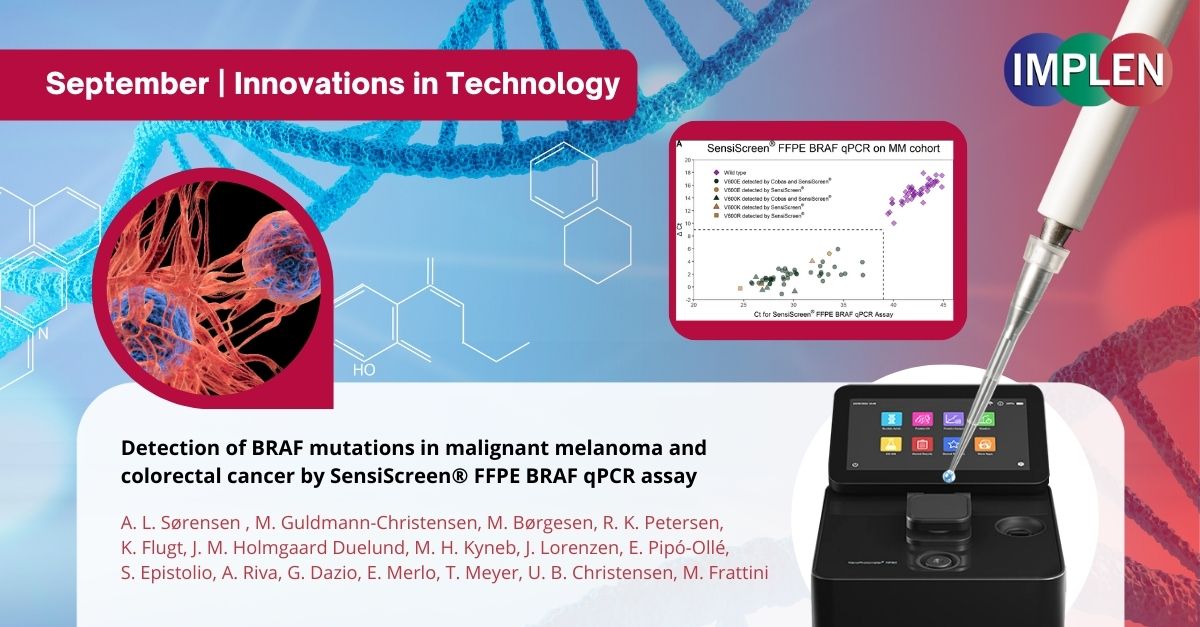
In the final issue, we are focusing on the detection of BRAF mutations in two types of cancer: malignant melanoma and colorectal cancer. BRAF mutations are known to play a critical role in the development and progression of these cancers, and detecting these mutations is crucial for treatment strategy. In a study led by Anna Lahn Sørensen and her team, an innovative screening assay was introduced, which can identify specific mutations in the BRAF gene. The SensiScreen® FFPE BRAF qPCR Assay uses a novel real-time PCR-based method for BRAF mutation detection based on PentaBases proprietary DNA analogue technology designed to work on standard real-time PCR instruments to serve as a highly sensitive and specific molecular diagnostic technique.This assay is designed to work with formalin-fixed, paraffin-embedded (FFPE) tissue samples, which are commonly used in clinical settings for preserving tissue specimens. This novel assay could provide valuable insights into the prevalence of BRAF mutations in these cancer types and enhance the accuracy of diagnostic testing in clinical laboratories. The SensiScreen® assay's sensitivity and specificity in detecting BRAF mutations in FFPE samples could enhance the accuracy of diagnostic testing in clinical laboratories. This could lead to more precise and timely diagnoses for patients, allowing for quicker initiation of appropriate treatments ultimately benefiting patients by guiding treatment decisions and potentially leading to better outcomes. The NanoPhotometer® was used in this work to measure the DNA concentrations. |
©2023 Implen. All rights reserved.