Implen Journal Club | February Issue |
|
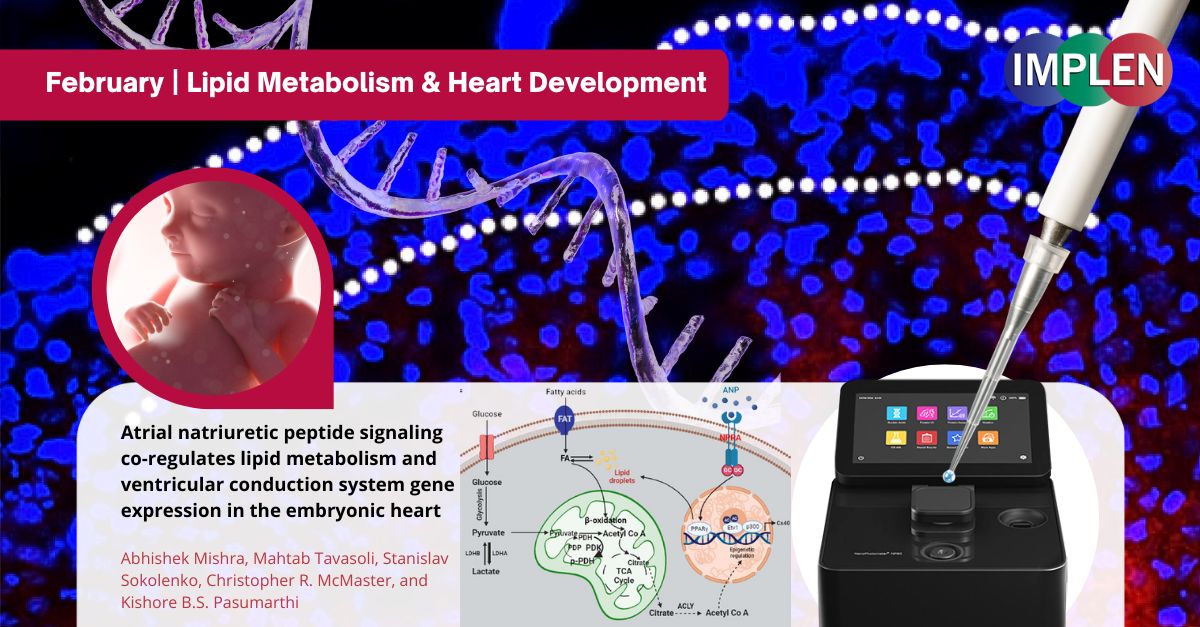
Heartstrings and Lipid Metabolism: Unlocking the Secrets of Embryonic Heart Development with ANP and Seahorse TechnologyIn anticipation of Valentine's Day, this week's Implen NanoPhotometer Journal Club is excited to showcase a captivating study by Mishra et al., focusing on the essential role of atrial natriuretic peptide (ANP) in embryonic heart development. The study specifically examined ANP's influence on lipid metabolism and the expression of genes within the ventricular conduction system (VCS). Employing the advanced capabilities of Agilent’s Seahorse XF Analyzer technology, the research offered a detailed analysis of ANP's effect on cellular metabolism, particularly highlighting its role in enhancing lipid metabolism. Through this work, ANP has been identified as a key factor in promoting the accumulation of lipid droplets, the oxidation of fatty acids (FAO), and the upregulation of the VCS marker gene Cx40 in embryonic ventricular cells. Such findings underscore the critical importance of lipid metabolism in the differentiation and maturation of VCS cells, which are vital for the proper electrical signaling and functionality of the heart. This study not only deepened our understanding of the mechanisms behind heart development but also opened up potential new avenues for therapeutic interventions targeting heart conduction disorders. By leveraging Seahorse technology, this research vividly illustrates the dynamic relationship between peptide signaling, lipid metabolism, and heart development, offering profound insights into the metabolic underpinnings of embryonic heart maturation. The Implen NanoPhotometer® was used in this study to quantify RNA. RNA samples with 260:280 absorbance ratio > 1.8 were used for further gene expression experiments. |
|
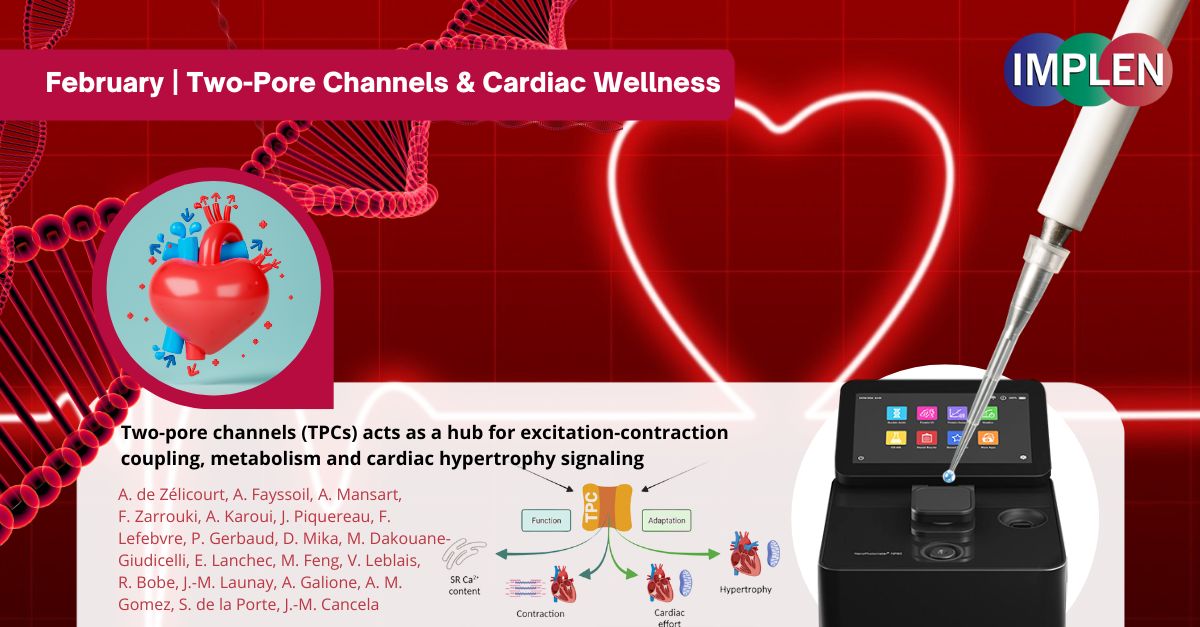
Valentine's Day Special: Love, Hearts, and the Hidden Role of Two-Pore Channels in Cardiac WellnessNext, Implen NanoPhotometer Journal Club is celebrating this Valentine's Day with a study by Zelicourt et. al. highlighting the heart's complex biology by exploring the indispensable role of two-pore channels (TPCs) in cardiac function. Utilizing mice models lacking TPC isoforms 1 and 2, this research revealed how the absence of these channels leads to significant alterations in left ventricular performance, both in terms of contraction and relaxation, without affecting the heart muscle cells' structure. This work revealed diminished contractile capability and sarcoplasmic reticulum Ca2+ content, underscoring calcium handling issues critical for heart contractions. TPC deletion's impact extended to the downregulation of vital proteins and metabolic regulators, highlighting a broader effect on cardiac metabolism and protein synthesis, compromising the heart's ability to fulfill heightened energy requirements. Additionally, the observation of heightened TPC expression in conditions of cardiac hypertrophy suggests these channels play a role in the heart's response to stress or damage, potentially offering a pathway for therapeutic intervention. This study demonstrated TPCs central to the integration of signals for excitation-contraction coupling, energy metabolism, suggesting their potential as therapeutic targets and marking a significant direction for future cardiac therapy and metabolic regulation research. The Implen NanoPhotometer® N120 was used in this study to quantify the total isolated RNA. |
|
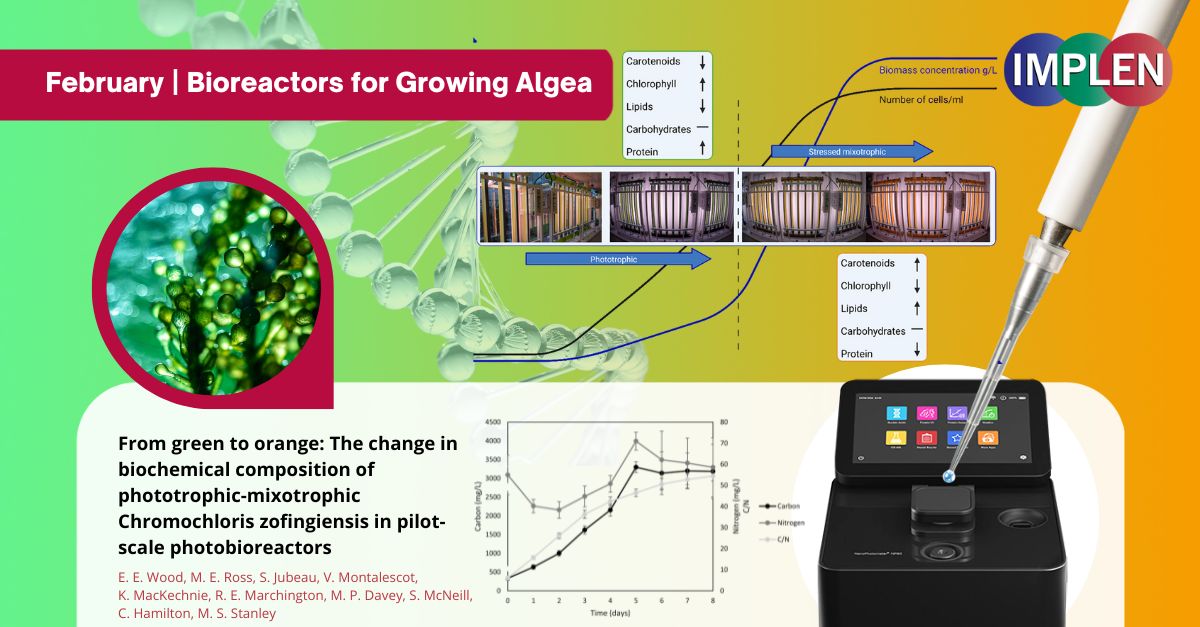
Growing Super Algae in Bioreactors: A New Way to Harvest Nature's Super PigmentThis issue is highlighting a study that investigated growing a type of algae called Chromochloris zofingiensis on a large scale to produce astaxanthin, a valuable compound often used in supplements and as a natural food colorant. This study found that this algae could be a better source for astaxanthin than the commonly used Haematococcus algae because it grows faster and produces more astaxanthin in a given space. Phyco-Lift photobioreactors (65L) were used for cultivating Chromochloris zofingiensis, focusing on the transition from green phase to orange phase cultures to enhance astaxanthin production. The algae were initially grown under normal light conditions and then given extra food (glucose) and kept in continuous light to boost their growth and astaxanthin production. It was discovered that not only did the algae produce more astaxanthin, but also produced more proteins, sugars, and fats, which are valuable for various uses.The study demonstrated that it's possible to grow this algae on a large scale for commercial astaxanthin production, which could lead to more sustainable and efficient production methods for this valuable compound. For growth and morphology assessment, the optical density of the cultures was measured using the Implen NanoPhotometer® at 750 nm. These tools played significant roles in the experimental setup by providing precise measurements essential for assessing the growth characteristics of Chromochloris zofingiensis and the successful induction of astaxanthin production. The precise control and monitoring offered by these instruments were vital. |
|
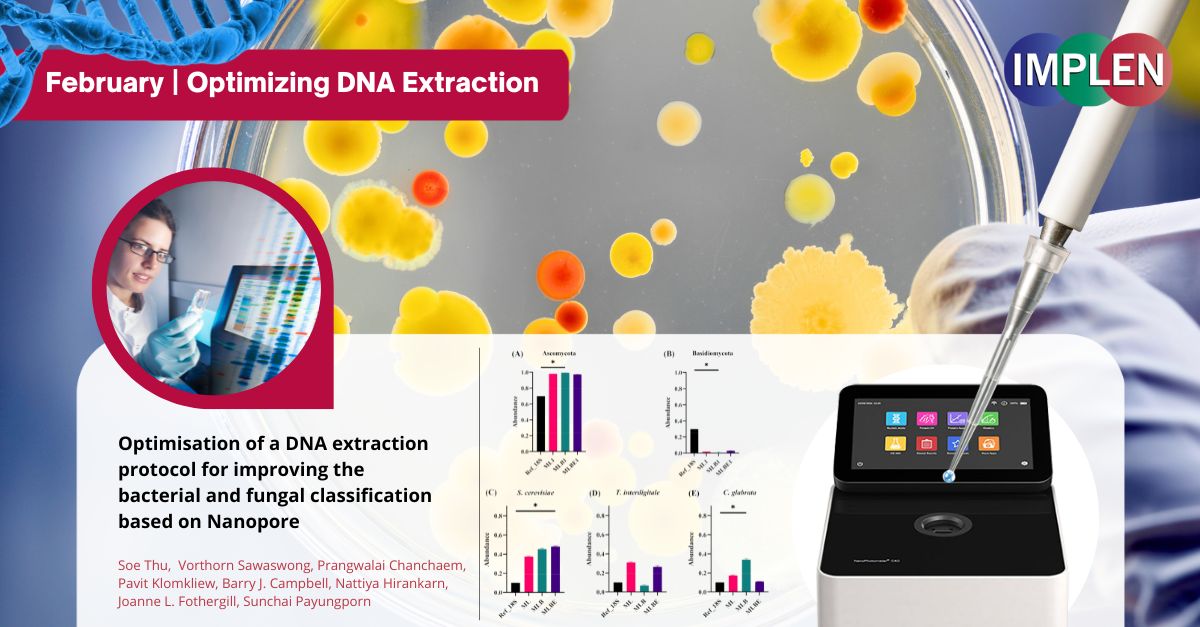
Optimizing DNA Extraction for Accurate Microbial Profiling with Oxford Nanopore Long-Read SequencingThe last issue is showcasing a recent study by Thu et al. to evaluate DNA extraction methods for analyzing bacterial and fungal communities using NanoPore long-read sequencing. Three methods were compared: 1) Lysis buffer only (ML), 2) Lysis buffer plus bead-beating (MLB), 3) Lysis buffer, bead-beating, and enzyme treatment (MLBE). DNA yields were similar, but MLB showed more variation between replicates. For identifying bacteria, MLB showed differences in abundances of certain groups compared to the reference, while ML and MLBE were more accurate. For fungi, MLB failed to detect some species present in the mock sample and altered the ratio of two major fungal groups. Overall taxonomic composition and diversity analyses revealed ML and MLBE had higher similarity to the reference data for both bacteria and fungi compared to MLB. This study demonstrated that bead-beating risks DNA shearing and is not recommended for long-read sequencing. The ML method alone is suggested as optimal considering DNA quality, accurate identification, cost, and processing time for simultaneous bacterial and fungal profiling. The NanoPhotometer® C40 was used to measure the quantity and quality of eluted DNA. |
©2024 Implen. All rights reserved.