Implen Journal Club | March Issue |
|
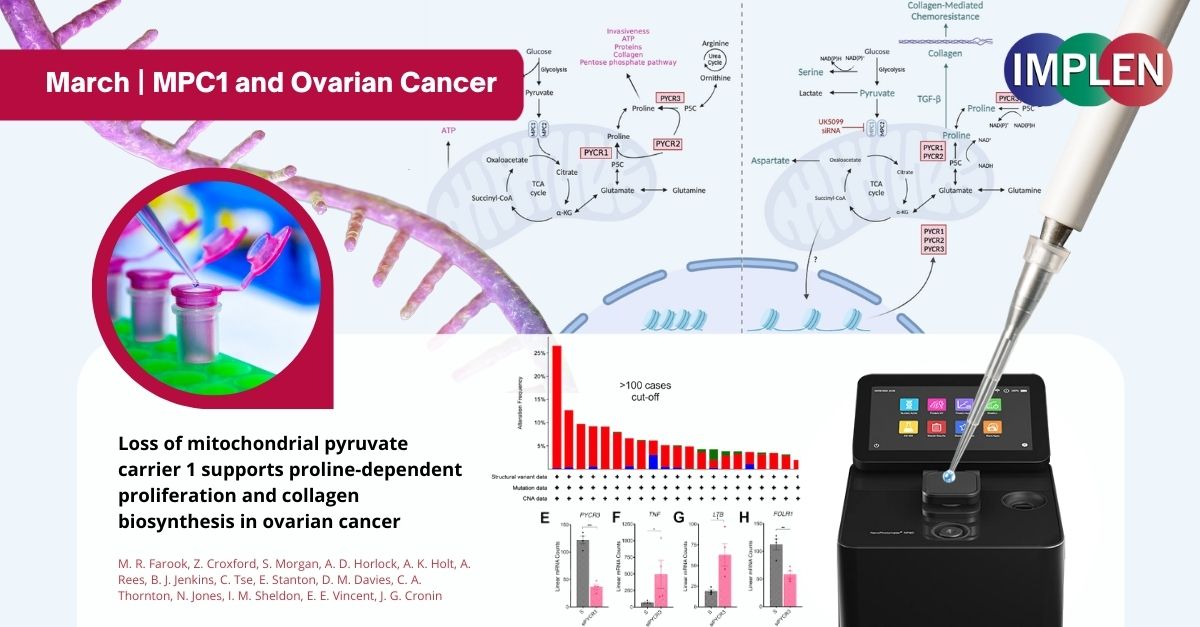
Unlocking the Metabolic Mysteries of Ovarian Cancer: The Role of MPC1 and New Therapeutic PathwaysThe first issue of the Implen NanoPhotometer® Journal Club is exploring the influence of MPC1, a protein typically acting against cancer growth, on ovarian cancer. In severe ovarian cancer cases, MPC1 levels drop, leading to poorer patient outcomes. In a study published by Farook et al. this month in the journal of Molecular Pathways, the Seahorse XF Bioanalyzer technology was utilized to investigate how cancer cells adapt their metabolism without MPC1. It was discovered that in the absence of MPC1, cancer cells shift to using glutamine for energy, aiding their growth and survival in adverse conditions. Additionally, the reduction of MPC1 increases the activity of enzymes responsible for producing proline, an amino acid crucial for cell growth and collagen development, thereby promoting cancer cell proliferation and the formation of aggressive tumors. By combining Seahorse technology with a nuanced analysis, this study reveals how ovarian cancer cells adjust their metabolic pathways due to MPC1 depletion. This insight opens the door to new therapeutic strategies that target these metabolic changes to combat ovarian cancer effectively. The NanoPhotometer® was used in this work to determine the RNA quality and concentration. |
|
Revolutionizing Drug Monitoring: How Magnets and Microneedles Are Changing the Game for Medication Tracking in Real TimeNext, the Implen NanoPhotometer® Journal Club is exploring the groundbreaking research by Reynoso et. al. that introduced a novel approach for conducting pharmacokinetic studies in live rodents. This method utilized 3D-printed, aptamer-based microneedle sensor arrays equipped with a magnetic attachment system, enabling the painless and precise monitoring of drug concentrations in the interstitial fluid just beneath the skin's surface. The secure magnetic placement ensured that the sensors remained firmly attached, overcoming previous stability issues. This research primarily examined the pharmacokinetics of tobramycin, an antibiotic used against Gram-negative bacteria, revealing insights into its absorption and effectiveness through innovative magnetic attachment technology. Notably, variations in the observed drug concentrations across different subjects were linked to the method of drug delivery, the hydration status of the rats, and genetic differences. The innovative magnetic attachment strategy not only promised to revolutionize measurements in research settings, potentially extending to awake animal subjects, but also suggested future avenues for non-invasive human health monitoring, especially in discrete body regions such as the ear lobes. Nonetheless, this study highlighted areas for refinement, such as improving the microneedles' sharpness for enhanced skin penetration and optimizing sensor design for greater accuracy. Future endeavors were set to focus on these improvements and delve deeper into the pharmacokinetic insights gained, aiming to advance our understanding of health and disease management. The concentration of aptamer solutions was measured using UV-Vis spectroscopy employing the Implen Nanophotometer® NP80. |
|
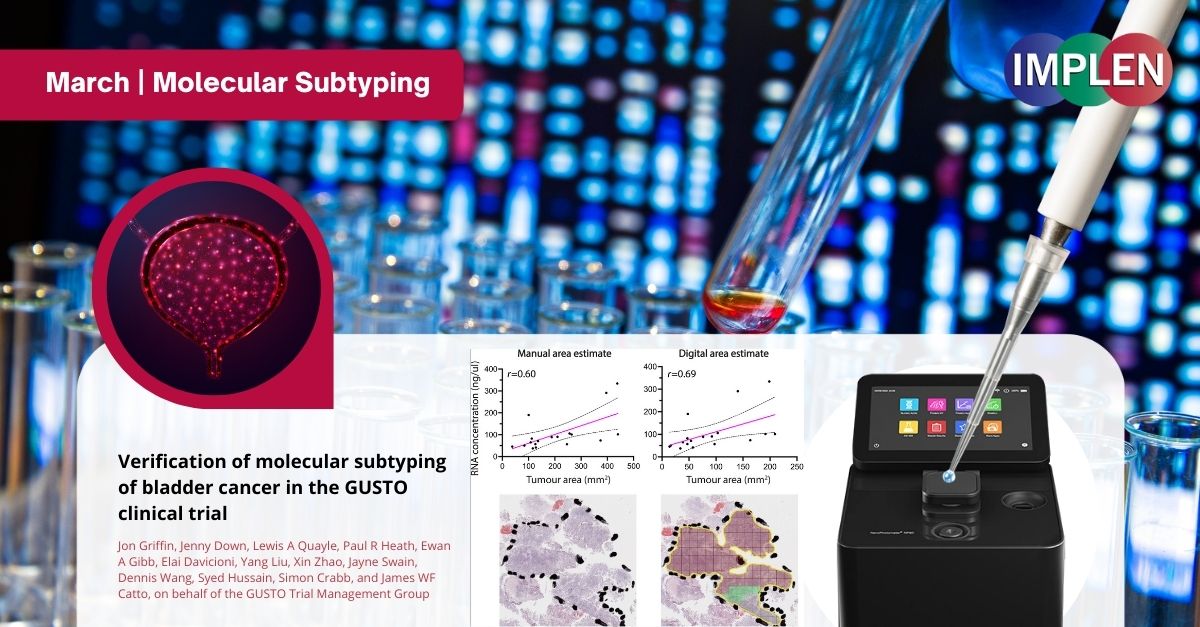
Advancing Bladder Cancer Treatment: GUSTO Trial's Molecular Subtyping Verification Study Shows Promising Path ForwardThis issue is delving into the GUSTO clinical trial that represents a significant step forward in the personalized treatment of muscle-invasive bladder cancer (MIBC) by utilizing molecular subtyping to guide neoadjuvant therapies. In the study published by Griffin et. al., a critical component of the approach involved pre-trial verification of the Decipher Bladder subtyping platform developed by Veracyte, to ensure its reliability and effectiveness when applied in an external trial laboratory setting. This study involved the analysis of 18 formalin-fixed paraffin-embedded MIBC samples using gene expression microarrays to assess the platform's performance. The findings of the verification study were highly promising, demonstrating successful RNA extraction of sufficient quality and quantity from all samples, which covered the entire spectrum of MIBC subtypes. The study confirmed both the intra- and inter-laboratory reproducibility of the subtyping platform, with no significant discrepancies observed in the classification of cancer subtypes across different laboratories. This result was crucial for the GUSTO trial, as it underlines the robustness and reliability of molecular subtyping by gene expression profiling for guiding treatment decisions. By demonstrating the feasibility and reliability of molecular subtyping in a clinical trial setting, this verification study paves the way for the GUSTO trial to explore new avenues in the personalized treatment of MIBC, potentially leading to improved outcomes for patients. The NanoPhotometer® was used in this work to determine the quality and quantity of RNA and cDNA samples. |
|
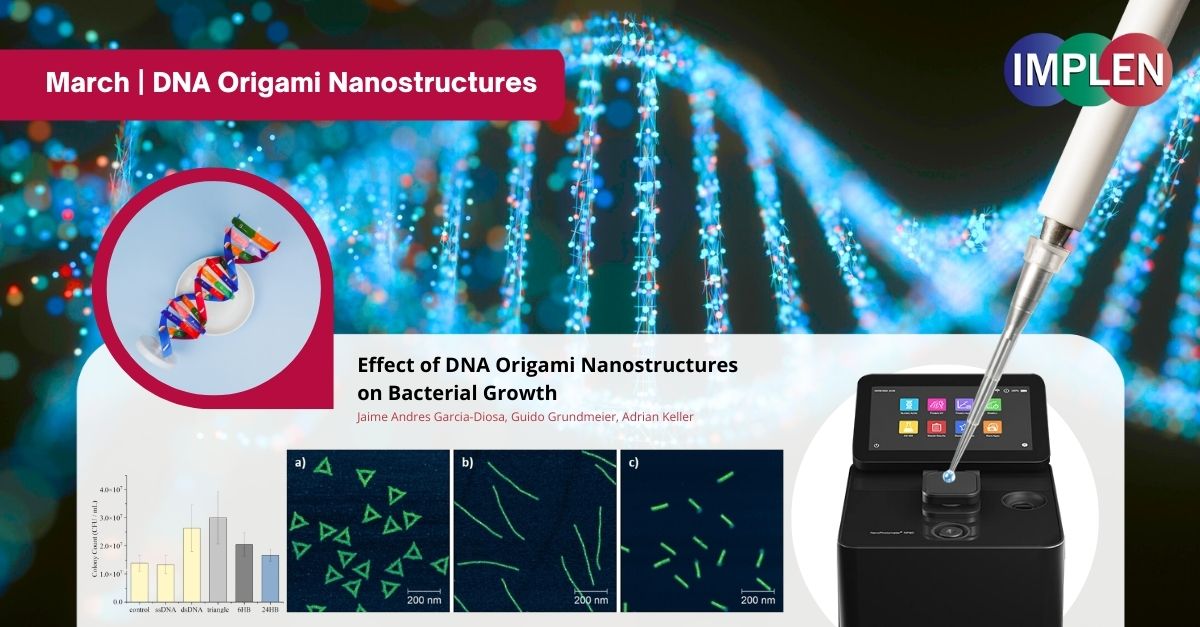
Shaping the Future of Antimicrobial Strategies: Unveiling the Impact of DNA Origami Nanostructures on Bacterial GrowthThe last issue is highlighting research recently published by Garcia-Diosa et al. investigating the interaction between DNA origami nanostructures and bacterial growth, focusing on Escherichia coli (Gram-negative) and Bacillus subtilis (Gram-positive) bacteria. DNA origami, which is both safe for the body and breaks down naturally, is being studied for its potential in medicine, especially in fighting infections. Three DNA origami shapes were examined: triangles, six-helix bundles (6HBs), and 24-helix bundles (24HBs), to assess their impact on bacterial growth. The experiments revealed a differential response between the bacterial species. E. coli utilized triangles and 24HBs as nutrient sources, promoting population growth, though less effectively than genomic DNA. No significant effect on E. coli growth was observed with 6HBs. Conversely, B. subtilis showed no significant growth variation when exposed to any of the DNA origami shapes. This indicates that the effect of DNA origami on bacterial growth is influenced by the bacterial species' competence signals and uptake mechanisms, as well as the shape and superstructure of the DNA origami. This study underscores the necessity of considering these factors in the development of antimicrobial DNA origami nanostructures, highlighting the complex interplay between nanostructures and bacterial cells. These insights are pivotal for leveraging DNA origami in antimicrobial applications, suggesting that shape-dependent interactions could inform the design of targeted antimicrobial strategies. The DNA origami concentration was determined by UV/Vis absorption using the Implen Nanophotometer®. |
©2024 Implen. All rights reserved.