OD600 (Cell Density, Bacterial Growth, Yeast Growth)
Technical Specs |
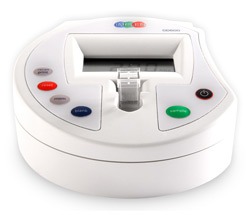
Implen Diluphotometer™ |
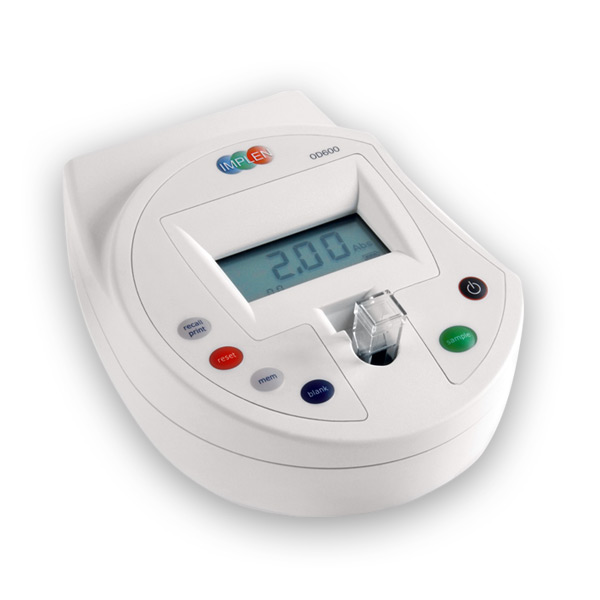
Implen OD600® |
| Main Applications | Optical density at 600 nm (OD600) | Optical density at 600 nm (OD600) & Turbidity standards (McFarland) |
| Photometric Accuracy | < ±0.05 @ 1A |
±0.01 @ 1A
±0.1 @ 0-8 McF |
| Photometric Range | –0.3 – 1.99 A | 0 – 4 A |
| Photometric Repeatability | < ±0.02 @ 1 A | ±0.002 @ 1A ±0.05 @ 0 – 8 MFU |
| Spectral Bandwidth | 40 nm | 18 nm |
| Optical Height | 8.5 mm | 8.5 mm |
| Operation |
LCD Display 6 Buttons |
3.5″ glove-compatible touchscreen – easy and quick to clean |
| Languages | English |
English, Chinese, French, Spanish, Japanese, German |
| Compatible Vessel Types |
10 mm macro- and semi-micro cuvettes 14, 16 mm round tubes DiluCell™ 10 |
10 mm macro- and semi-micro cuvettes DiluCell™ 10 |
| Onboard Memory | 99 readings | 4 GB internal storage – > 500,000 measurements |
| Data Export | Via Software Tool; Formats: Excel |
USB 2.0 A – Flash Drive; Formats: Excel and .csv (for LIMS: JSON) |
| Connectivity | RS232 | LIMS integration via WiFi or Ethernet/Lan |
| Internal Rechargeable Battery | Yes | Yes |
| Dimensions | 180 x 150 x 60 mm | 140 x 110 x 70 mm |
| Weight | 0.6 kg | 0.6 kg |
| No longer available | Purchase |
Learn more about our new Implen OD600® device offering a higher concentration range, accuracy and reproducibility, featuring a 3.5″ glove-compatible display, user-friendly interface, utmost compatibility without the need for adapters, large data storage, universal capabilities, and effortless data export.
The OD600 Basics
Optical Density (OD) measurements of microbial and cell growth are one of the most common methods used in a microbiology lab. Some of the main applications are the determination of the optimal time at which to harvest, the determination of the optimal time to induce a culture when running a protein expression protocol or the monitoring of cloning procedures.
The growth of cells, bacteria or yeast (cell density, bacterial growth, yeast growth) in liquid culture media is commonly controlled by measuring the optical density at 600 nm (OD600). OD600 measurements are typically used to determine the stage of growth of a bacterial culture, these measurements help ensure that cells are harvested at an optimum point that corresponds to an appropriate density of live cells.
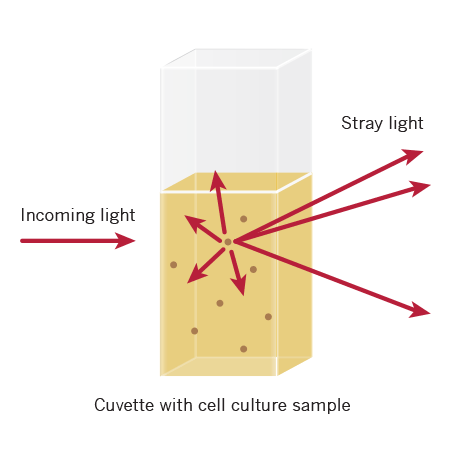
Since optical density in the case of OD600 measurements results from light scattering rather than light absorption, size and shape as well as dead cells and debris of a cell may add to light dissipating. Distinctive cell types that are at densities of the same level (eg. cells/mL) may therefore show varying values OD600 when estimated on a similar instrument.
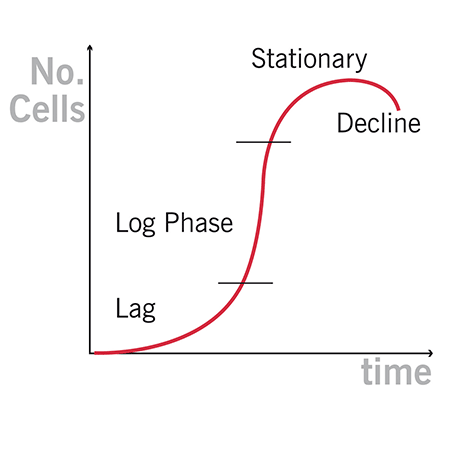
Growth of bacterial cells typically progresses through a series of consecutive phases including lag, log, stationary, and decline.
In general, cells should be harvested towards the end of the log phase, using the optical density of the samples to determine when this point has been reached. Cells are routinely grown until the absorbance at 600 nm (known as OD600) reaches approximately 0.4 prior to induction or harvesting.
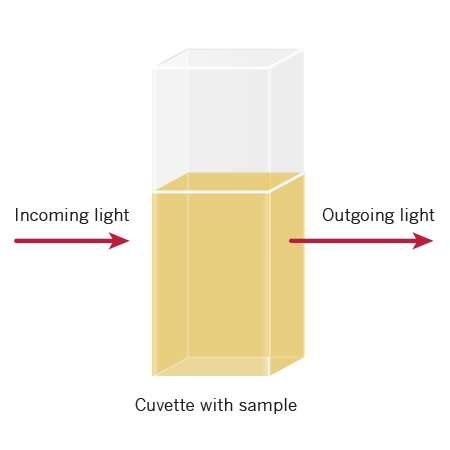
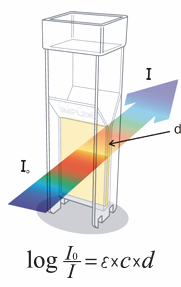
The Beer-Lambert Law and OD600
The Beer-Lambert Law, also known as Beer’s Law, empirically relates the absorption of light to the properties of the sample. This law states that there is a logarithmic relationship between the transmission of light through a specific sample (T = I/Io with I = outgoing light and Io = incoming light), the molar extinction coefficient for a specific compound (ε), the concentration of the absorbing species in the material (c) and the distance the light travels (d).
OD estimations have gotten synonyms with estimations of bacterial concentration (C) or number (N), as per the Beer-Lambert law. However measurements of OD are actually measurements of turbidity, we can therefore apply the Beer-Lambert law, with certain contemplations, just for microbial colonies of low densities.
Difference in OD600 Readings
For turbid samples such as cell cultures, the major contributor for the absorbance measured is light scattering and not the result of molecular absorption following the Beer-Lambert Law. The measurements are therefore depending on the optical setup of the spectrophotometer (distance between the cell holder and instrument exit slit, monochromator optics, slit geometry, etc.), different instrument types will most likely tend to give different OD 600 readings for the same turbid sample. Therefore, if results from different spectrophotometers are to be compared, they must be normalized first by either a simple correlation of the OD readings using the same sample on the two different instruments to compare
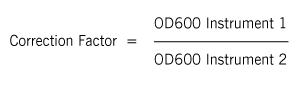
A calibration curve can be constructed by comparing measured OD 600 to expected OD 600 over a range of different concentrations. Expected OD 600 is determined by counting cell number using an alternative technique (for example microscope slide method) and converting to OD 600 using the rule of thumb that 1 OD 600 = 5 x 108 cells/ml for E. coli. A high-quality instrument like the NanoPhotometer® or the OD600 DiluPhotometer™ comes with a “correction factor” of 1 as default, which, once adjusted will help to provide comparable results between different equipment.
The use of disposable cuvettes is recommended rather than micro/nano volume technologies (measurements in a drop) for optical density measurements of cell culture solutions. The amount of cells is reflected in the reading and the likelihood of fluctuating amount of cells in a drop from sample to sample can be considered as extremely significant. It is therefore recommended to use cuvettes since the amount of error in a bigger volume is not as significant. The cuvette measurements provide a bigger average and therefore more reproducible readings. Also, to prevent the suspension settling too quickly and giving an OD reading that changes with time, glycerol should be added to the sample.
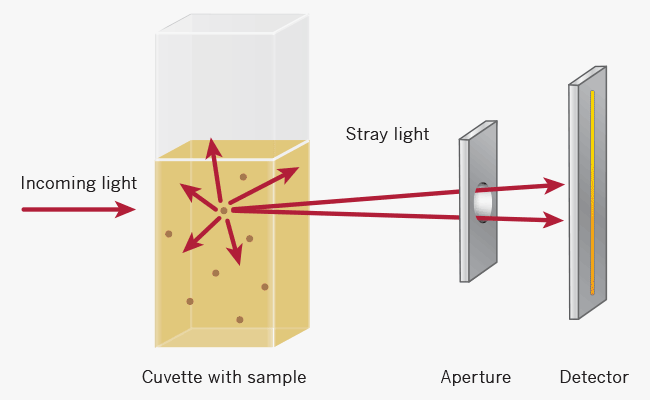
Higher absorbance reading
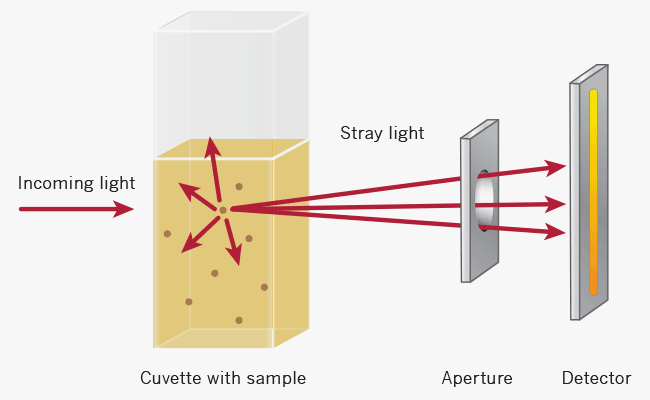
Lower absorbance reading
Linear Range for OD600 Measurements

The Beer-Lambert Law and OD600
The linear range of the instrument used for the measurements is critical to cover the entire growth cycle of the culture, an OD limit of at least 2.5 OD is advised to provide sufficient working range. Please bear in mind that the sample may not be linear over the entire range if a high amount of dead cells are present, obscuring the result. If samples should still be out of range, dilutions may become necessary; the parameters should be carefully selected to cover the entire desired area without compromising the linearity of the setup.
To reduce the required volume for the OD600 measurement and to avoid time-consuming and error-prone manual dilutions, special cuvettes are available for the researcher. DiluCell™ allows for lower sample volume requirements and provides automatic virtual sample dilution. Due to the unique design of DiluCell™ cuvettes, the light-path is reduced from the standard 10 mm to 1.0 mm (DC 10). According to the Lambert Beer Law, this shortened pathlength results in an automatic sample dilution by factor 10 (DC 10). Automatic dilution with DiluCell™ saves time and excludes dilution errors and cross contaminations.
OD600 Tool
Click on the link below to access a spreadsheet OD600 Tool, Version 1.2, that helps generate microbial growth curves according to OD600 values as well as to calculate other values of interest for microbiology experiments like cell forming units CFU/mL or time of inoculation. Once instrument calibration and standard curve have been established, it can easily be used to document growth experiments for any type of cell culture.
Implen GmbH
Implen, based in Munich, Germany, is a leading supplier for innovative spectroscopy instruments and consumables for the non-destructive analysis of liquid samples offering a complete range of spectrophotometers for cuvette and drop measurements.
|
|
|
|
The OD600 Basics
Optical Density (OD) measurements of microbial and cell growth are one of the most common methods used in a microbiology lab. Some of the main applications are the determination of the optimal time at which to harvest, the determination of the optimal time to induce a culture when running a protein expression protocol or the monitoring of cloning procedures.
The growth of cells, bacteria or yeast (cell density, bacterial growth, yeast growth) in liquid culture media is commonly controlled by measuring the optical density at 600 nm (OD600). OD600 measurements are typically used to determine the stage of growth of a bacterial culture, these measurements help ensure that cells are harvested at an optimum point that corresponds to an appropriate density of live cells.
Since optical density in the case of OD600 measurements results from light scattering rather than light absorption, size and shape as well as dead cells and debris of a cell may add to light dissipating. Distinctive cell types that are at densities of the same level (eg. cells/mL) may therefore show varying values OD600 when estimated on a similar instrument.
Growth of bacterial cells typically progresses through a series of consecutive phases including lag, log, stationary, and decline.
In general, cells should be harvested towards the end of the log phase, using the optical density of the samples to determine when this point has been reached. Cells are routinely grown until the absorbance at 600 nm (known as OD600) reaches approximately 0.4 prior to induction or harvesting.




The Beer-Lambert Law and OD600
The Lambert-Beer-Law, also known as Beer’s Law, empirically relates the absorption of light to the properties of the sample. This law states that there is a logarithmic relationship between the transmission of light through a specific sample (T = I/Io with I = outgoing light and Io = incoming light), the molar extinction coefficient for a specific compound (ε), the concentration of the absorbing species in the material (c) and the distance the light travels (d).
OD estimations have gotten synonyms with estimations of bacterial concentration (C) or number (N), as per the Beer-Lambert law. However measurements of OD are actually measurements of turbidity, we can therefore apply the Beer-Lambert law, with certain contemplations, just for microbial colonies of low densities.
Difference in OD600 Readings
For turbid samples such as cell cultures, the major contributor for the absorbance measured is light scattering and not the result of molecular absorption following the Beer-Lambert Law. The measurements are therefore depending on the optical setup of the spectrophotometer (distance between the cell holder and instrument exit slit, monochromator optics, slit geometry, etc.), different instrument types will most likely tend to give different OD 600 readings for the same turbid sample. Therefore, if results from different spectrophotometers are to be compared, they must be normalized first by either a simple correlation of the OD readings using the same sample on the two different instruments to compare
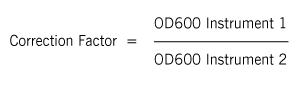
A calibration curve can be constructed by comparing measured OD 600 to expected OD 600 over a range of different concentrations. Expected OD 600 is determined by counting cell number using an alternative technique (for example microscope slide method) and converting to OD 600 using the rule of thumb that 1 OD 600 = 5 x 108 cells/ml for E. coli. A high-quality instrument like the NanoPhotometer® or the OD600 DiluPhotometer™ comes with a “correction factor” of 1 as default, which, once adjusted will help to provide comparable results between different equipment.
The use of disposable cuvettes is recommended rather than micro/nano volume technologies (measurements in a drop) for optical density measurements of cell culture solutions. The amount of cells is reflected in the reading and the likelihood of fluctuating amount of cells in a drop from sample to sample can be considered as extremely significant. It is therefore recommended to use cuvettes since the amount of error in a bigger volume is not as significant. The cuvette measurements provide a bigger average and therefore more reproducible readings. Also, to prevent the suspension settling too quickly and giving an OD reading that changes with time, glycerol should be added to the sample.

Higher absorbance reading

Lower absorbance reading
Linear Range for OD600 Measurements

The Beer-Lambert Law and OD600
The linear range of the instrument used for the measurements is critical to cover the entire growth cycle of the culture, an OD limit of at least 2.5 OD is advised to provide sufficient working range. Please bear in mind that the sample may not be linear over the entire range if a high amount of dead cells are present, obscuring the result. If samples should still be out of range, dilutions may become necessary; the parameters should be carefully selected to cover the entire desired area without compromising the linearity of the setup.
To reduce the required volume for the OD600 measurement and to avoid time-consuming and error-prone manual dilutions, special cuvettes are available for the researcher. DiluCell™ allows for lower sample volume requirements and provides automatic virtual sample dilution. Due to the unique design of DiluCell™ cuvettes, the light-path is reduced from the standard 10 mm to 1.0 mm (DC 10). According to the Lambert Beer Law, this shortened pathlength results in an automatic sample dilution by factor 10 (DC 10). Automatic dilution with DiluCell™ saves time and excludes dilution errors and cross contaminations.
OD600 Tool
Click on the link below to access a spreadsheet OD600 Tool, Version 1.2, that helps generate microbial growth curves according to OD600 values as well as to calculate other values of interest for microbiology experiments like cell forming units CFU/mL or time of inoculation. Once instrument calibration and standard curve have been established, it can easily be used to document growth experiments for any type of cell culture.
Implen GmbH
Implen, based in Munich, Germany, is a leading supplier for innovative spectroscopy instruments and consumables for the non-destructive analysis of liquid samples offering a complete range of spectrophotometers for cuvette and drop measurements.
|
|
|
|