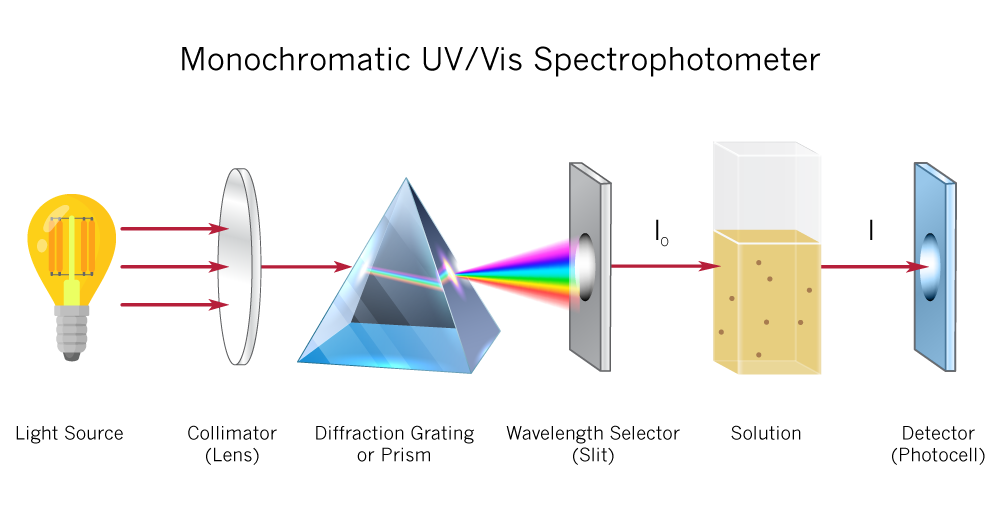
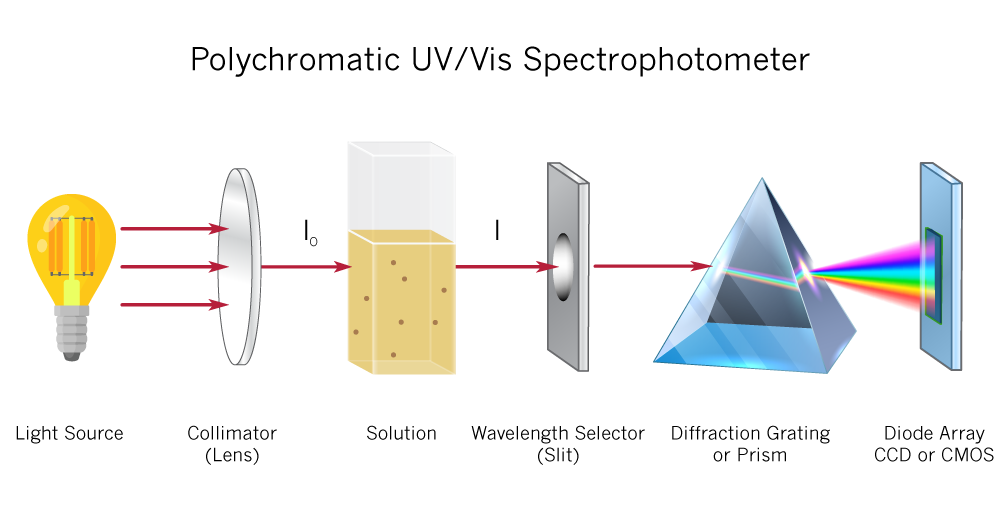
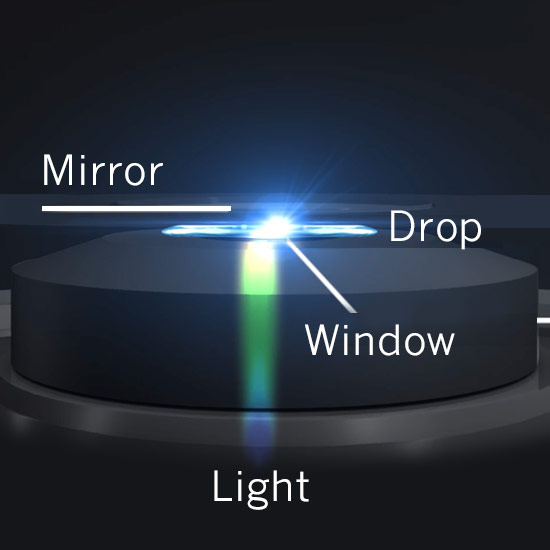
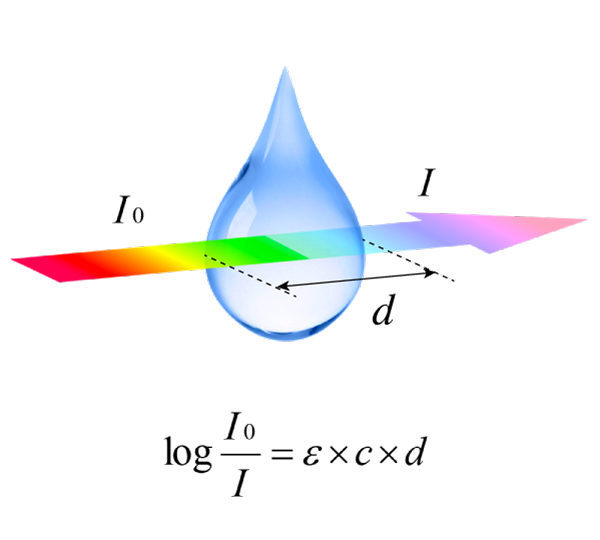
The absorption of light is based on interaction of light with the electronic and vibrational state of a molecule.
Each type of molecule has an individual set of energy levels associated with its chemical bonds and therefore will absorb light of specific wavelengths showing unique spectral properties.
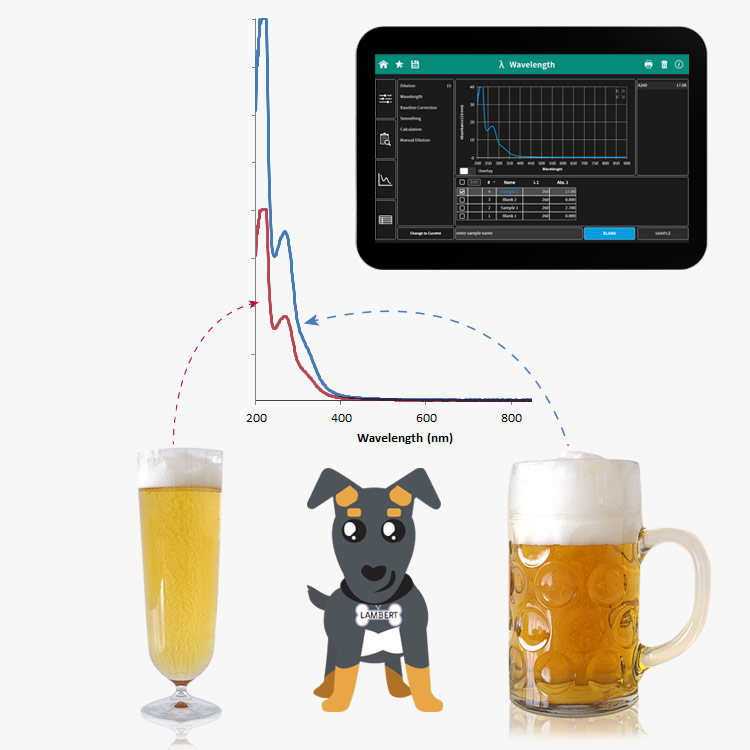
To run an experiment on the NanoPhotometer®, he only needs to swipe a small drop of beer and put it on the microvolume pedestal. Since daylight is considered white light (an overlay of the three basic colors red, green and blue – RGB) and beer is yellow, it can be expected that it absorbs light in the blue range and only lets pass green and red light (which when overlapped results in yellow to our eyes)… and voila: the scan confirms what we have anticipated perfectly!